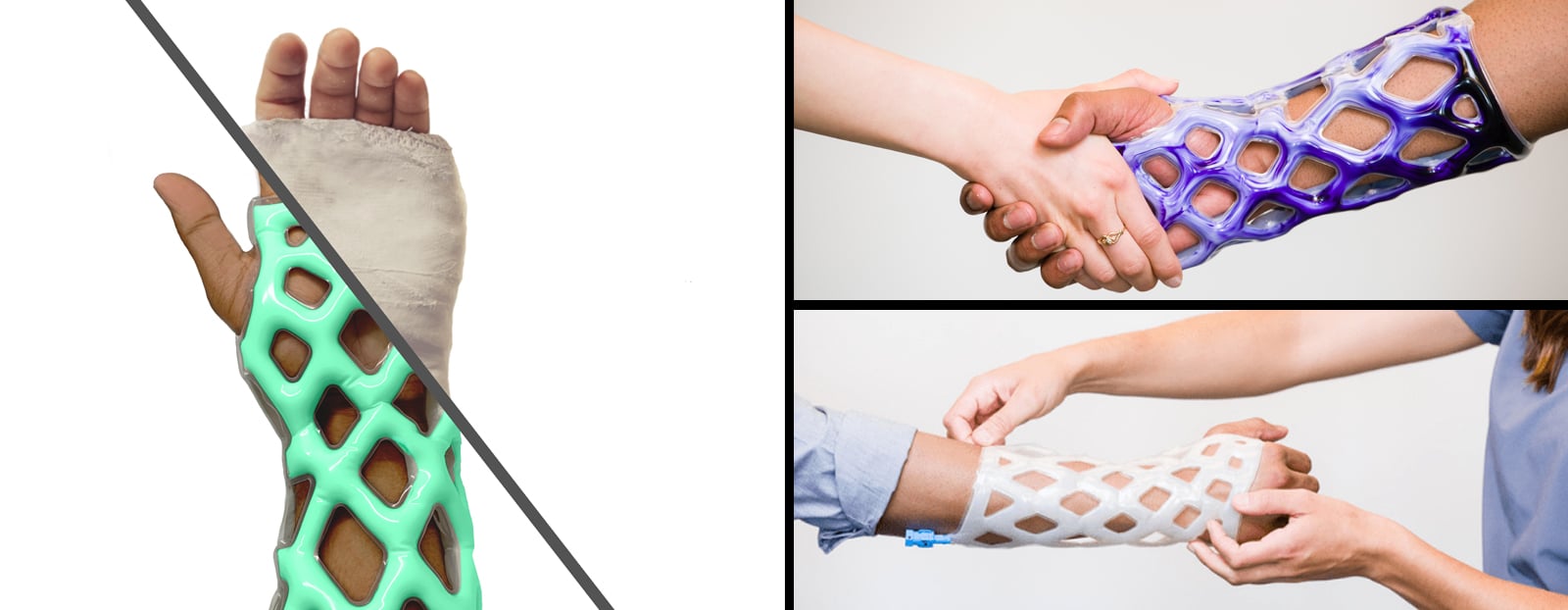
Chicago-based medical startup, Cast21 is developing a new cast and brace alternative for treatment of orthopedic injuries that is waterproof, breathable, lightweight, and easily applied and removed. To do this, their design includes a multi-layer sleeve that is injected with a two-part epoxy which flows into the device as a liquid, and cures as a solid in an exothermic reaction. The result is a fast-deploying, color customizable cast which maximizes patients’ quality of life during the healing process.
However, to successfully develop this new replacement for a decades old solution requires a lot of multidisciplinary engineering. In the case of Cast21, the technical road map includes a wide variety of Structural, Fluid, and Thermal challenges. This presents the ideal opportunity to deploy Computer Aided Engineering (CAE) Simulation… but how?
Simulation Assessment
Before any Simulation software solution is implemented, an assessment of the project, and most importantly, the qualification test program is conducted to look for the best areas where simulation can help. Remember, a simulation is just a digital replication of the actual analog test, so before spending big time and money on simulation, the test program should be as defined as possible.
For this, we look at the technical challenges, the risk to the business, and the costs involved. By looking at the big picture, we can move forward with the confidence that we are investing in Computer Aided Engineering in the right areas. For Cast21, who is a young, dynamic company disrupting a massively regulated industry, a simulation assessment focuses on the entire process from manufacturing and distribution, to patient comfort and safety.

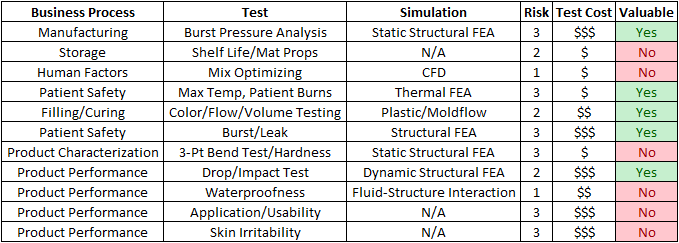
For intellectual property reasons, we cannot dive too deep into the details, but for the purposes of walking through the process, Cast21 has agreed to share the following Simulation Assessment table that Fastway Engineering helped fill out with their engineering team.
Digging into the Details
Business Process: Here we step through the manufacturing and distribution processes to look for areas of potential defects. The most organized way to do this is to start with the manufacturing processes, and end up with product performance & reliability, and other customer facing characteristics. Of course with Medical products, product performance and patient safety is paramount.
Test: In this column category, we review the types of tests that are readily available for evaluating the product for potential defects. This can range from product-specific and industry-specific tests, to standard tests documented by the American Society of Materials and testing (ASTM). For Cast21, the most critical test is the filling/curing process, which includes a lot of variables that must be controlled.

Simulation: When working with Cast21 for this part, we used our expertise in the CAE landscape to present the various software tools that can effectively simulate the required test. This starts with simple FEA simulations like Static Structural and Steady State Thermal Analysis, to Dynamics (both structural and fluid), and ultimately to some of the most complex cutting edge Fluid-Structure Interaction (FSI) capabilities. In some cases, such as patient comfort, there are no simulation solutions that can replicate the necessary testing.
Risk: No assessment is complete without an assessment of the level of risk that is associated to a potential defect. For this we used a simple scale of 1 to 3.
- A level 1 risk is the lowest level. A defect in this area could be managed by regular, existing, processes with little impact to the business.
- A level 2 risk is significant risk. Fixing the defect would require significant money and resources, but could be done. It is undesirable, but possible.
- A level 3 risk is critical, and a defect at this level would put the business at risk. An example of a level 3 risk in the medical industry is anything related to patient safety.
Test Cost: By sending out Request for Quotes (RFQ’s) we obtain the out-of-pocket costs associated with conducting the tests. This is the most quantifiable category, as it is possible to get hard numbers from 3rd party service providers. These costs are added to internal costs of conducting the test, such as prototyping and engineering technical support. If the test is destructive, such as with some material property tests, the prototype costs are unrecoverable even if something goes wrong with the test.
Valuable: Finally, we put all of our data together to assess how valuable it would be to leverage Simulation in addition to testing. In this case, using a simple yes/no, we identify tasks/potential defects that have:
- Significant or Critical Risk
- High Test Cost
- Readily available Simulation solutions.
Once the Assessment is done, the real work begins. Having the confidence to know where to spend time, such as building the right mesh for the right application, allows the Cast21 team to spend their time and resources in the best place possible - moving the project forward.
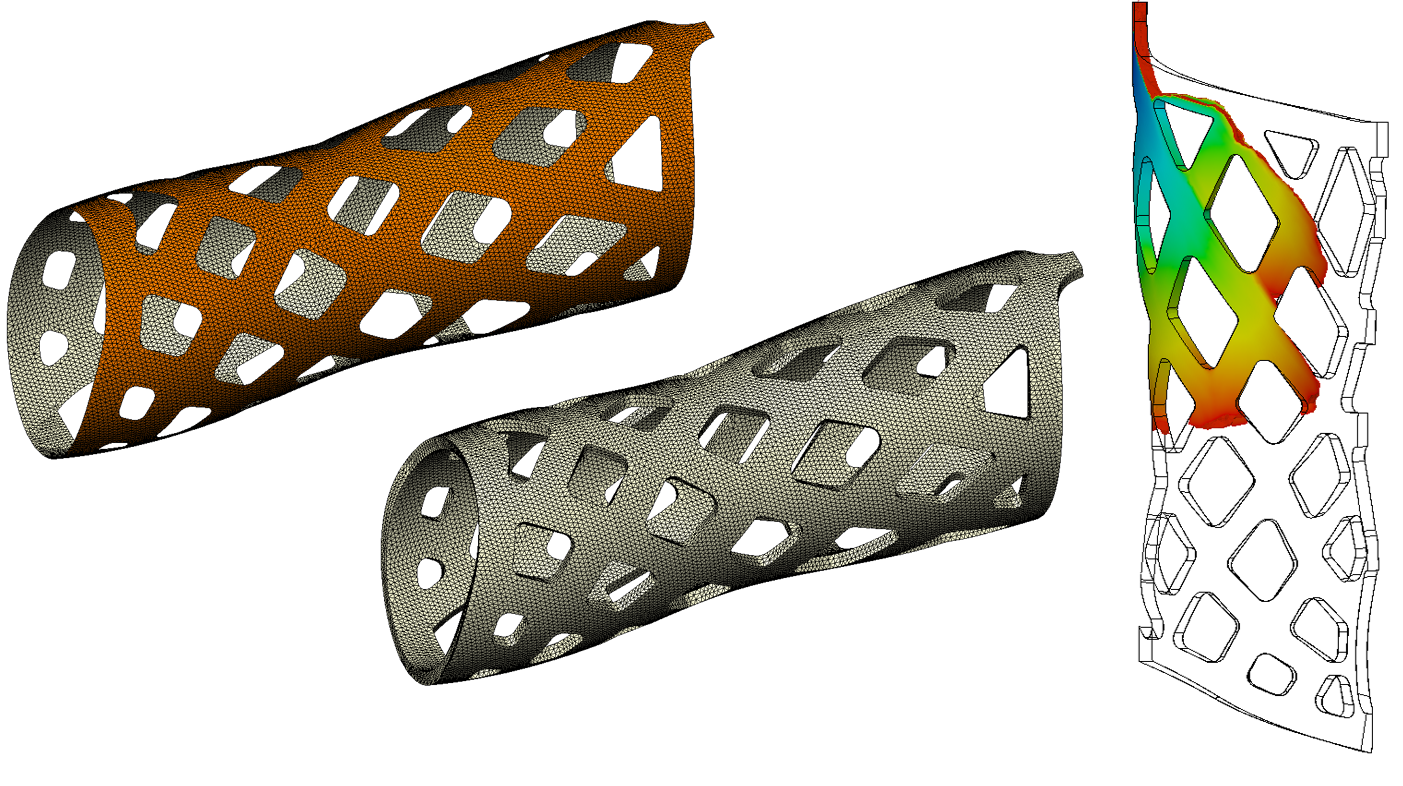
Here we see a simplified shell mesh used for a Structural FEA model, a 3D mesh needed for an accurate Transient Thermal FEA Model, and the results of a Sleeve Filling analysis using CFD.
Establishing an Effective Simulation Program
As can be seen above for Cast21, simulation is valuable for 5 of the 11 highlighted tasks. From here, a simulation program was scoped with a focus on Static & Dynamic Structural FEA, Thermal Analysis, and CFD Flow Analysis. With a defined simulation scope, software licenses/capabilities can be vetted, and costs can be associated to the simulation program. There are two important things to keep in mind at this stage:
- Simulation program costs go in 3 categories: hardware, software, and training. Successful simulation programs include proper budgeting for all 3 categories. At Fastway, we often see training get under-budgeted, and the result is always an increase in engineering effort, and a push back of project deliverable due dates.
- If Simulation is being deployed within an engineering organization for the first time, it will not completely replace testing. In most cases, testing is significantly reduced, but only after the proper Verification and Validation procedures have been followed (i.e. your simulation results correlate with your test data within, say, 5%). In the mean time, simulation benchmarking is done in parallel with testing so simulation errors can be identified and reduced. Resources and funding must be allocated accordingly.
Finally, as is typical in the medical industry, there are a number of mandatory patient tests (called "clinical trials") which cannot be replaced with simulation. For Cast21, this means testing out their innovative product in the field to assess for patient usability and performance. At the time of this article, Cast21 is conducting clinical trials. Doctors who are interested in learning more about Cast21 can go here: https://www.cast21.com/medical-professionals/ and patients can learn more here: https://www.cast21.com/patients/.
Are considering using simulation for your project, but are unsure how to deploy it effectively? Contact us for a free Simulation and Project Risk Assessment.